June 2009 Vol. 236 No. 6
Features
Selection Of Pipe Material For Low-Temperature Service
The selection of material for any specific environment is directly dependent on the material’s properties, especially those properties that are affected by that special environment.
Metal properties are classified in terms of Mechanical, Physical and Chemical properties. These are further subdivided into Structure Sensitive or Structure Insensitive properties. The following table describes these properties.
Table 1: Metal properties.

In this article, we are concerned only with the structure-sensitive mechanical properties of metal. Metals are favored as a construction material because they offer a combination of mechanical properties that are unique and not found among non-metals. Metals are generally strong and many can be loaded or stressed to very high levels before breaking. One property of metals of interest is their capacity to exhibit a high degree of elastic behavior in their early load-carrying capacity. This is a very important property for effective use of the metal as a construction material. When these metals are loaded beyond their elastic range they exhibit another set of important properties called ductility and toughness. These properties and how they are affected by change in temperature are the point of this article.
Pipeline Steels
We will focus on carbon and low-alloy steels. It may be noted that the bulk of the material that is used in conventional pipeline engineering comes from this generic group. Aptly, it is the ductility and toughness of these metals and how they are affected by the variation of temperature that is our subject. The emphasis is made on the variation under low temperature. For this purpose it is essential to know what is meant by these metal properties and by low temperature. The following definitions are understood by fracture mechanics.
Ductility is defined as the amount of plastic deformation that metal undergoes in resisting the fracture under stress. This is a structure-sensitive property and is affected by the chemical composition.
Toughness is the ability of the metal to deform plastically and absorb energy in the process before fracturing. This mechanical and structure sensitive property is the indicator of how the given metal would fail at the application of stress beyond the capacity of the metal, and whether that failure will be ductile or brittle. Only one assessment of toughness can be made with some reasonable accuracy from ordinary tensile testing, and that is the metal displays either ductile or brittle behavior. From that it can be assumed that the metal displaying little ductility is unlikely to display a ductile failure if stressed beyond its limits. The failure in this case would be brittle.
The temperature of metal is found to have profound influence on the brittle/ductile behavior. The influence of higher temperature on metal behavior is considerable. The rise in temperature is often associated with increased ductility and corresponding lowering of the yield strength. The rupture at elevated temperatures is often intergranular, and little or no deformation of the fractured surface may have occurred. When lowered below room temperature, the propensity for brittle fracture increases.
ASTM E 616 defines some of the terminology associated with Fracture Mechanics and Testing, such as:
- The term fracture is strictly defined as irregular surface that forms when metal is broken into separate parts. If the fracture has propagated only part way in the metal and metal is still in one piece, it is called a crack.
- A crack is defined as two coincident-free surfaces in a metal that join along a common front called the crack tip, which is usually very sharp.
- The term fracture is used when the separation in metal occurs at relatively low temperature and metal ductility and toughness performance is the chief topic.
- The term rupture is more associated with the discussion of metal separation at elevated temperatures.
As noted previously, two basic types of fracture occur in metals: ductile and brittle. These two modes are easily recognized when they occur in exclusion, but fractures in metal often have mixed morphology and that is aptly called mixed mode. The mechanisms that initiate the fracture are shear fracture, cleavage fracture, and intergranular fracture. Only the shear mechanism produces ductile fracture. It may be noted that like the modes discussed here, the failure mechanisms also have no exclusivity.
A crack is defined above as two coincident-free surfaces in a metal that join along a common front called the crack tip, which is usually very sharp. Irrespective of the fracture being ductile or brittle, the fracture process is viewed as having two principal steps:
1. Crack initiation, and
2. Crack propagation.
Knowledge of these two steps is essential as there is a noticeable difference in the amount of energy required to execute them. The relative level of energy required for initiation and for propagation determines the course of events which will occur when the metal is subjected to stress.
There are several aspects to the fracture mechanics that tie in with the subject of metal ductility and toughness but this article is not planned for detailed information on fracture mechanics. Hence, these are not discussed in detail but some specific-related topics are listed in Table 2.
Table 2: Topics related to fracture mechanics.
- Effects of axiality of stress,
- Crack arrest theory,
- Stress intensity representation,
- Stress gradient,
- Rate of Strain,
- Effect of Cyclic Stress,
- Fatigue Crack,
- Crack Propagation, (KIc= ? ??a)
- Griffith’s theory of fracture mechanics,
- Irwin’s K = ?E x G,
- Crack Surface Displacement Mode,
- Crack Tip Opening Displacement (CTOD), (BS 5762-1979 and BS 7448 part-I)
- R-Curve Test methods
- J- Integral Test method,
- Linear-Elastic Fracture Mechanics (LEFM) (ASTM E 399),
- Elastic-Plastic Fracture Mechanics (EPFM),
- Nil Ductility Temperature (NDT).
Though the topics in Table 2 are not commonly taken into consideration when selecting suitable material for an onshore pipeline, these are essential parts of subsea pipeline and riser technology. In fact, some of the specification (e.g. API 1104, DNV-OS F101 etc.) suggest the use of fracture mechanics to determine the failure behavior of metal in these services.
Returning to our earlier discussion, lowering the temperature of metal profoundly affects fracture behavior. Strength, ductility, toughness and other properties are changed in all metals when they are exposed to temperature near absolute zero. The properties of metals at very low temperatures are of more than casual interest because pipelines, welded pressure equipment and vessels are expected to operate satisfactorily at levels below room temperatures. For example, moderate sub-zero temperatures are imposed on equipment for dewaxing petroleum and for storage of nitrogen, liquefied fuel gases and pipelines.
Much lower temperatures are involved in cryogenic services where metal temperature falls to –100 C (-150 F) and below. The cryogenic service may involve storage of liquefied industrial gases like oxygen and nitrogen. Toward the very bottom of the temperature scale, there is a real challenge for metals that are used in the construction of equipment for producing and containing liquid hydrogen and liquid helium,because these elements in liquefied form are increasingly important in new technologies. Helium in liquefied form is only slightly above absolute zero, which is 1 Kelvin (-273.16 C or – 459.69 F).
Absolute zero (1 K) is the theoretical temperature at which matter has no kinetic energy and atoms no longer exhibit motion. Man has yet to cool any material to absolute zero, so it is unknown how metals would behave when cooled to this boundary condition.
However, metal components have been brought to the temperatures very close to absolute zero, hence it presents a special challenge to metals and welded components as they would be required to serve in this extremely low temperature.
When cooled below room temperature every metal will reach a temperature where the kinetic energy will be reduced to nil. The atoms of the element will move closer and the lattice parameters will become smaller. All these changes would affect the mechanical properties of the metal.
Metal Strength At Low Temperature
As we have seen, as temperature is lowered from room temperature, 75oF (24oC or 297oK), to absolute zero, 1oK, the atoms of an element move closer together by dimensions easily compounded from the coefficient of thermal expansion. Several changes occur as a result of this smaller lattice parameter. For example, the elastic module increases. In general, the tensile strength and yield strength of all materials increase as the temperature is lowered to the nil ductility temperature (NDT) , where the yield and tensile strength are equal (?o = ?u). The change in these properties is variable in degree for different metals but change does occur.
When the temperature of low-carbon or low-alloy steel is lowered, the corresponding increase in strength of metals occurs. This is attributed to an increase in resistance to plastic flow. Because plastic flow is strongly dependent upon the nature of the crystalline structure, it would be logical to assume that metals with the same kind of structure would react similarly.
A cautionary note: The material in ASTM A 333 Grades 1,3,4,6,9 and 10 is required to have minimum of 10 ft-lbs absorbed energy (impact values).This is the same as ASTM A 350 LF1, but material ASTM A 350 LF2 and LF3 are required to have minimum of 12 ft-lbs absorbed energy (impact values). This is at any given temperature, respective of that material.
Selecting Material From Specification And Codebooks
There are several ASME/ASTM specifications specifically tailored for low-temperature services, but it is important to check if the specified test temperatures for the metal in use is in tally with the design temperature of the system. ASTM-A/ASME -SA105 is not a low-temperature material; however, it may be used for low temperature if all the other factors are conforming to the requirements and an additional impact test on the material is carried out at a temperature that is in tally with the design temperature.
Similarly, ASTM A 106 pipes (grade A, B or C) must be checked for the test temperatures because ASTM A 106 is specified as “high-temperature” material and rightfully the impact test is not even included in the non-mandatory requirement. The same is the case with ASTM A 105 forged material discussed above. Concerning ASTM A 333 grades 1, 3, 4, 6, 9 and 10 pipes for the acceptable impact values and their test temperatures, the specification must be referenced before arbitrarily using them for any service temperature range. ASTM A 350 LF1 (-20 F), LF2 (-50 F), LF 3 (-150 F) are suitable for low-temperature service to the limits set by the specification, but one should check the specified energy absorption value Cv to ensure it is in tally with the system design parameters.
An informed selection has to be made. There are several boiler-quality plate materials specified by the ASTM specifications and ASME codes but not all are suitable for low-temperature services. Some are so designed metallurgically that they are not suitable for low-temperature service. Plate material conforming to the ASTM A 515 specification is an example. Most of the metals that are fit for low temperature are generally tested to 32oF (0 C) unless specified otherwise. So, the general assumption that all ASME material is good up to -20oF will not be correct, unless it is tested and material test report so declares.
API mandates that PSL2 pipes be tested at 32oF (0 C) or any lower temperature as agreed between the buyer and manufacturer and is expected to have 20 ft-lbf (27 J) absorbed energy. The same is not true for PSL1 pipes. In either case, it is important to determine what was the actual test temperature and what responsibility engineers have to ensure that the test temperature is in tally with the design temperature of the system.
Among pipeliners, a question is often raised if, in designing a buried pipeline, one needs to consider the low temperature. The answer is not metallurgical since it is unrelated to the material property as much as it is geographical and environmental, that is, the design conditions. The data provided by the user (clients) and the specification must be consulted.
Generally, a buried pipeline will not be subject to very low temperatures unless buried in permafrost, so no specific caution beyond the general design considerations would be required. However, the general guidance in such case should be to look at the product properties, risk analysis, product leakage, and will a reduction in pressure at a certain point reduce the temperature to what is considered a low-temperature range.
If there is a cause to expect lower temperature, then determine to what extent lower temperature will occur during the life of service. If the temperature is ever in the critical low range, it will be prudent to identify those conditions and take them into account while selecting the material.
Similar consideration applies to the aboveground pipe and components. Aboveground valves flanges and pipes are more exposed to the weather and are also carrying the similar product. Therefore, they have greater propensity to face low temperature in their service lives. The following questions must be asked and answered: Are they insulated? Are they heated? Is there any possibility of depressurization that would lead to extensive temperature reduction, etc? There is a multiplicity of factors that affect the understanding of the material behavior in extreme stress conditions. All possible factors must be identified and addressed.
Conclusion
The questions we have tried to explore are more complex than this discussion which is an attempt to simplify the basic understanding of the subject. This discussion is intended to bring out the importance of the subject and direct readers to available resources for material selection issues.
Important Additional Information
The sub-ambient temperature dependence of yield strength ?o (Rp0.2) and ultimate tensile strength ?u in a bcc metal is shown in Figure 1. Consider the graph, the material is ductile until a very low temperature, point A, where Y.S. equals the UTS of the material (?o = ?u). Point A represents the NDT temperature for a flaw-free material. The curve BCD represents the fracture strength of a specimen containing a small flaw (a < 0.1mm). The temperature corresponding to point C is the highest temperature at which the fracture strength ?f ? ?o. Thus point C represents the NDT for a specimen with a small flaw. 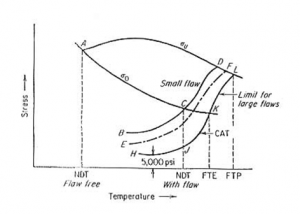 The presence of a small flaw raises the NDT of steel by about 200°F (110°C). Increasing the flaw size decreases the fracture stress curve, as in curve EF, until with increasing flaw size a limiting curve of fracture stress HJKL is reached. Below the NDT the limiting safe stress is 5,000-8,000 psi (~35 to 55 MPa). Above the NDT the stress required for the unstable propagation of a long flaw (JKL) rises sharply with increasing temperature. This is the crack-arrest temperature curve (CAT). The CAT curve defines the highest temperature at which unstable crack propagation can occur at any stress level. Fracture will not occur for any point to the right of the CAT curve. The temperature above which elastic stresses cannot propagate a crack is the fracture transition elastic (FTE). The temperature defines the FTE, at the point K, when the CAT curve crosses the Yield Strength, ?o curve. The fracture transition plastic (FTP) is the temperature where the CAT curve crosses the Ultimate Tensile Strength ?u curve (point L). Above this temperature, the material behaves as if it is flaw-free, for any crack, no matter how large, cannot propagate as an unstable fracture. Author
The presence of a small flaw raises the NDT of steel by about 200°F (110°C). Increasing the flaw size decreases the fracture stress curve, as in curve EF, until with increasing flaw size a limiting curve of fracture stress HJKL is reached. Below the NDT the limiting safe stress is 5,000-8,000 psi (~35 to 55 MPa). Above the NDT the stress required for the unstable propagation of a long flaw (JKL) rises sharply with increasing temperature. This is the crack-arrest temperature curve (CAT). The CAT curve defines the highest temperature at which unstable crack propagation can occur at any stress level. Fracture will not occur for any point to the right of the CAT curve. The temperature above which elastic stresses cannot propagate a crack is the fracture transition elastic (FTE). The temperature defines the FTE, at the point K, when the CAT curve crosses the Yield Strength, ?o curve. The fracture transition plastic (FTP) is the temperature where the CAT curve crosses the Ultimate Tensile Strength ?u curve (point L). Above this temperature, the material behaves as if it is flaw-free, for any crack, no matter how large, cannot propagate as an unstable fracture. Author
Ramesh Singh is a senior principal engineer for Gulf Interstate Engineering, 16010 Barkers Point Lane, Houston, TX 77079. He specializes in materials, welding and corrosion. He graduated from California Coast University with a master of science degree (2003) in engineering management and gained his basic metallurgical education (1984) from Air Force Technical Institute in India. He is registered with the Engineering Council in the UK and is a member of The Welding Institute, Cambridge UK. He is a NACE member and has served as secretary and vice chair of the NACE Houston chapter. rsingh@gie.com.





Comments