June 2012 Vol. 239 No. 6
Features
Important Properties For Industrial Gas Turbine Fuels

The quality and composition of fuel burned in a gas turbine impacts the life of the turbine, particularly its combustion system and turbine section. The fuel specified for a given application is usually based on availability and price. Natural gas is a typical fuel of choice for gas turbines due to its low cost, widespread availability, and low resulting emissions. Lately, compression requirements in shale gas fields have drawn attention to the capability of gas turbines to burn fuels with higher than normal levels of heavier hydrocarbons. Engines with lean premix emissions control systems require thorough mixing of the fuel with air in the fuel injectors prior to combustion. Therefore, a combustible mixture exists in the premixing portion of the injector, and ignition of this mixture prior to entering the combustor must be prevented. In this article, we discuss different properties of fuel that have to be considered when qualifying a fuel for a combustion system.
The amount of energy that will be released during the combustion process for a specific gaseous fuel composition is described by the heating value. For simple cycle gas turbines, the lower heating value is generally used since the latent energy of the water vapor in the exhaust is not recovered. An important parameter related to the lower heating value is the Wobbe index:

(Eq. 1)
If two different fuel gas compositions have the same Wobbe index, the pressure drop in a given fuel system will be the same for both gases and in general direct substitution is possible and no change to the fuel system is required. The Wobbe index is thus an indication of energy flow in the system at the same gas pressure and pressure drop.
The Wobbe index is used as a parameter to indicate the ability of the overall fuel handling and injection system to accommodate the fuel composition. If the Wobbe index varies too far from the design value, changes to the fuel system need to be made. A high Wobbe index also often indicates the presence of heavier hydrocarbons in the fuel, while a low Wobbe index is often caused by the presence of significant amounts of noncombustible fuel components or by the presence of significant amounts of (highly combustible) hydrogen or carbon monoxide.
A good design criterion is that gases having a Wobbe index within ±10% can be substituted without making adjustments to the fuel control system or injector flow area. Both Lean-Premix and conventional combustion gas turbines can be designed to use the standard fuel with Wobbe index range of 1220 ±10%. This range would be typical of pipeline quality natural gas. Lean-Premix combustion systems in industrial gas turbines are usually designed for standard natural gas. The use with other gases usually requires careful evaluation and testing.
However, the Wobbe index does not capture the effects of other fuel properties. The entire fuel composition must also be considered and, if more reactive species such as hydrogen, alkenes and carbon monoxide are present in significant quantities, additional changes to the fuel system may be required.
The Dew point of a gas is a function of gas composition, pressure and temperature. It describes the boundary between the single phase gas and the two phase (gas and liquid) state of a fluid (Elliott et al, 2004). Usually, the water dew point of a fuel gas is reported separately from the hydrocarbon dew point. Heavy hydrocarbons, even if they are only present in traces, have a large impact on the dew point. In many instances, a gas analysis groups all the heavier hydrocarbons into one number (e.g. C6+). Since the dew point depends on the distribution of the heavy hydrocarbons, estimates can be made based on characterization methods (Campbell, 2000). However, these estimates are often not very accurate, and direct dew point measurements may be the preferred method.
Because most gases will see a reduction in temperature during isenthalpic expansion (this is the Joule-Thompson Effect), it is possible that even a dry gas can develop liquids if it is subject to the pressure drop in a typical fuel supply system (Figure 1). It is, therefore, necessary to provide fuel gas sufficiently superheated. Values of 28 degrees C/ 50 degrees F above dew point at turbine skid edge are frequently used as a requirement, but the appropriate amount (which can be higher or lower) of superheat can be determined by a detailed dew point analysis. The amount of superheat needs to include allowances for uncertainty in the fuel gas composition at present and in the future, as well as the potential for heat loss of the fuel system (Kurz et al, 2007).
Additional complications come from the fact that the combustor pressure (and with it the pressure drop in the fuel system) depend on the engine load.
Blowout refers to a situation where the flame becomes detached from the location where it is anchored and is physically “blown out” of the combustor. Blowout is often referred to as the “static stability” limit of the combustor. Blowout occurs when the time required for chemical reaction becomes longer than the combustion zone residence time. It can be an issue because the chemical kinetic rates and flame propagation speeds vary widely with fuel composition. For example, many candidate fuels have similar heating values but also have chemical kinetic times that vary by an order of magnitude.
The opposite problem is flashback, where the flame physically propagates upstream of the region where it is supposed to anchor and into premixing passages that are not designed for high temperatures. Similar to blowout, flashback is an issue because of the widely varying flame speeds of candidate fuels. Again, fuels with similar heating values but with flame speeds that vary by factors of five or more are common (Lieuwen et al, 2006). Flashback occurs when the turbulent flame speed exceeds the flow velocity along some streamline, allowing the flame to propagate upstream into the premixing section. Flashback often occurs in the flow boundary layer, since this is the point of lowest flow velocity. As such, the effect of fuel composition variations upon flashback depend upon the corresponding change in turbulent flame speed. Flame speed is a propagation of the flame front moving in the combustion zone. Changes in the fuel composition, fuel to air ratio and inlet temperature affect the flame speed (Glassman, 1996).
The laminar flame speed, also called flame velocity, or burning velocity, is defined as the velocity at which unburned gases move through the combustion wave in the direction normal to the wave surface (Glassman, 1996). A key point here is that the flame speed does not vary linearly between the respective pure values of the mixture constituents. For example, the addition of H2 to CH4 does not have a significant impact upon the flame speed until H2 is the dominant constituent of the mixture. The impact of adding diluents, like CO2, is that the flame speed is still lower than the non-diluted mixtures, even if the temperature is maintained by increasing the equivalence ratio (Lieuwen et al, 2006).
However, most issues are related to the turbulent flame speed, which depends, besides the laminar flame speed, also on the turbulence levels of the gases in question. In particular, data show that as the turbulence intensity increases, the turbulent flame speed initially increases, then asymptotes to a constant value, and then at very high turbulence intensities begins to decrease. The most obvious effect of fuel properties on the turbulent flame speed is through the laminar flame speed. For example, for a given turbulence intensity and a given burner, fuels with higher laminar flame speeds should have higher turbulent flame speeds. However, turbulence intensity and laminar flame speed alone do not capture many important characteristics of the turbulent flame speed. Two different fuel mixtures having the same laminar flame speed, turbulence intensity, and burner can have appreciably different turbulent flame speeds, depending on the diffusion characteristics of the species involved (Lieuwen, et al, 2006).
Flame propagation velocity is also strongly influenced by the fuel/air mixture ratio; the leaner the mixture the lower the velocity. If the flow velocity exceeds the flame propagation velocity, then flameout could occur. If the flame propagation velocity exceeds the flow velocity, then flashback within the premixing injectors could occur that can cause damage by overheating the injector tips and walls. To maintain flame stability at a point, the velocity of the fuel-air mixture must be within the flame-propagation speed to prevent flashback (Lefebvre, 1998).
Flame flashback from the combustion chamber into the premixing zone is one of the inherent reliability problems of lean premixed combustion. The flame speed is one of the most important parameters governing flashback. High flame speeds occur for example in associated gases containing high percentages of propane or butane (Figure 2).
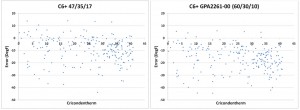
Figure 2: Fuel Type Effects on Flame Speed.
Autoignition is a process where a combustible mixture spontaneously reacts and releases heat in absence of any concentrated source of ignition such as a spark or a flame (Lefebvre, 1998). Rather than the flame propagating upstream into the premixing section, autoignition involves spontaneous ignition of the mixture in the premixing section. Similar to flashback, it results in chemical reactions and hot gases in premixing sections, but its physical origins are quite different from those of flashback. In lean premix combustors, or in general, in any combustor where fuel and air are premixed prior to combustion, this spontaneous ignition inside the injector barrel has to be avoided, because it can damage combustor components, and yields high pollutant emissions because combustion occurs before sufficient premix is achieved. The autoignition delay time of a fuel is the time required for a mixture to spontaneously ignite at some given condition. This parameter is a function of the fuel composition, the fuel to air ratio, the pressure, and the mixture temperature. Ignition delay time is of importance to the combustion specialist because it is a direct indication of potential autoignition in the mixing barrel, and it is a useful parameter that defines the chemical kinetic time scale at any temperature, such as in the main burner, thus, playing an important role in the position of the flame relative to the injector tip.
Leaner mixtures tend to have a longer delay time, while higher mixture temperatures and higher pressures tend to shorten the delay time. In a lean premix injector, the flow velocities thus have to be high enough to avoid autoignition inside the injector at the prevailing temperatures. Increasing the content of heavier hydrocarbons in an associated gas leads to a decrease of delay time. This is mainly caused by the non-symmetry of all higher hydrocarbons: Heavy hydrocarbons can be attacked much easier than methane molecules, resulting in reduced ignition delay times (Figure 3).
Figure 3: Autoignition Delay Time Depending on Fuel Constituents.
In general, the characteristic kinetic times decrease with the addition of hydrogen, with the lowest times (and hence faster chemical kinetics) corresponding to mixtures of CO and H2. For the ignition times (given in milliseconds) for 1000 K temperatures, the longest times (and hence slower chemical kinetics) are attributed to the mixtures containing mostly methane. Similar conclusions can be drawn for the higher temperature (1400 K) conditions. For example, CH4-only mixtures ignite at approximately 800 µs (microseconds) at 1400 K, while most H2- and CO-based mixtures ignite in less than 10 µs (Lieuwen, et al.). In general, little data is available for specific mixtures, and engine specific tests are often necessary to identify if there will be any issues.
Another important parameter is the ratio of flammability limits. In the combustor, the fuel and air must be continually burned to keep the engine running. When the flame in the combustor is extinguished it is called a flameout or blowout. The fuel to air ratio changes with the engine load, as described earlier. In order to prevent flameout, the combustor must support combustion over a range of fuel to air ratios. Each fuel composition has its own flammability range (ratio of flammability Limits). The ratio of flammability limits is defined as the upper flammability limit divided by the lower flammability limit. Knowing the ratio of flammability limits allows a decision to be made as to whether the fuel composition has a broad enough flammability range to support combustion for all operating points of the engine. The upper flammability limit is the maximum fuel percentage (volumetric) mixed with air that will still light and burn when exposed to a spark or other ignition source. The lower is the minimum fuel percentage to sustain combustion. Different gases have different ranges of flammability. As the fuel heating value decreases below standard levels, the torch igniter and the combustion system may require standard natural gas or liquid fuel for startup or shutdown, as well as possible restrictions on turbine transient load operation (Kurz et al., 2007).
The stoichiometric flame temperature impacts the amount of NOX emissions. It is also a parameter to help verify that a given fuel composition can be burned at all gas turbine operating loads and idle. Across the flammability range the mixtures of fuel and air will burn at different temperatures. As the fuel to air ratio is increased from the lower flammability limit, the flame temperature will increase. Upon further increase in the ratio a point will be reached where the amount of fuel and air will be perfectly matched so that all the oxygen in the air is reacted with all the fuel–this is called the stoichiometric fuel to air ratio. It also corresponds to the maximum flame temperature. As the fuel to air ratio is increased further still the flame temperature starts to decrease and continues to decrease until the upper flammability limit is reached. In standard combustion systems, with a very heterogeneous mixture, the flame temperature is close to the stoichiometric flame temperature. The flame temperature has a significant impact on the NOX production rate. Fuel gas with a high amount of non-combustibles will usually cause low flame temperatures, while fuel gas containing amounts of hydrogen, carbon monoxide, or heavier hydrocarbons, will exhibit higher flame temperatures. Therefore, a fuel gas with a high amount of diluents will yield lower NOX emissions even in standard combustion systems.
Combustion instability refers to damaging pressure oscillations associated with oscillations in the combustion heat release rate. These oscillations cause wear and damage to combustor components and, in extreme cases, can cause liberation of pieces into the hot gas path, damaging downstream turbine components. Combustion instability issues are highlighted here because the conditions under which they occur can be influenced by the fuel composition (Lieuwen, et al., 2006). A necessary, but not sufficient, condition for instability to occur is that the unsteady pressure and heat release oscillations must be in phase (or, more precisely, that their phase difference is less than ninety degrees). Fuel composition variations affect combustion instabilities by altering this phase angle.
In order to understand how variations in fuel composition affect the phase difference between pressure and heat release fluctuations, it is necessary to consider the specific mechanism responsible for the instability. Two mechanisms are known to be particularly significant in premixed systems: these are fuel/air ratio oscillations and vortex shedding. In the former mechanism, acoustic oscillations in the premixer section cause fluctuations in the fuel and/or air supply rates, thus producing a reactive mixture whose equivalence ratio varies periodically in time. The resulting mixture fluctuation is convected to the flame where it produces heat release oscillations that drive the instability. The coupling of the premixer acoustics with the fuel system is affected by the pressure drop across the fuel injector. The vortex shedding mechanism, as its name suggests, is due to large scale, coherent vertical structures. These structures are the result of flow separation from flame-holders and rapid expansions, as well as vortex breakdown in swirling flows. They are convected by the flow to the flame where they distort the flame front and thereby cause the rate of heat release to change. Fuel/air ratio oscillations and vortex shedding become important when the resulting heat release perturbation is in phase with the pressure fluctuation.
Fuel issues are not limited to combustion itself. Potential hazards associated with fuel, including flammability, detonation limits, and autoignition temperatures must be considered inside and around the engine in case of unintentional fuel leakage. Turbine enclosures are usually equipped with gas detectors, and the enclosure ventilation system is optimized to avoid accumulation of leaked gases. Low molecular weight gases, which rise rapidly, may be trapped in high dead spots in the enclosure, while heavy gases tend to accumulate on the ground or in low spots. In case of leakage, accumulation above the flammability limits must be avoided (Santon et al, 2002). For non-luminous gas fires (especially hydrogen), fire detection may be difficult.
Negative Joule Thompson coefficients will cause the fuel gas temperature to rise during isenthalpic expansion (for example through a leak), which may result in an explosion if autoignition temperatures are reached. This is a special concern with hydrogen rich fuels. Toxic gases, especially the odorless ones, require special safeguards against leakage.
In many systems, the gas composition and quality may be subject to variations. Typically, the major sources of contaminants within these fuels are: Solids, water, heavy gases present as liquids, lube oil from upstream compressors (especially reciprocating compressors), hydrogen sulfide, carbon dioxide, siloxanes (particularly in landfills). Carbon monoxide and hydrogen also need special consideration (Kurz et al, 2007).
This article is an attempt to describe different properties of gas fuel that have to be considered when qualifying a fuel for a combustion system. The impact of physical and chemical characteristics of gas fuels for gas turbines were linked with combustion characteristics and the resulting concerns.
Literature references are available on request to the authors or to Pipeline & Gas Journal.





Comments