March 2014, Vol. 241 No. 3
Features
Fusion-Bonded Epoxys Effects On Cathodic Disbondment
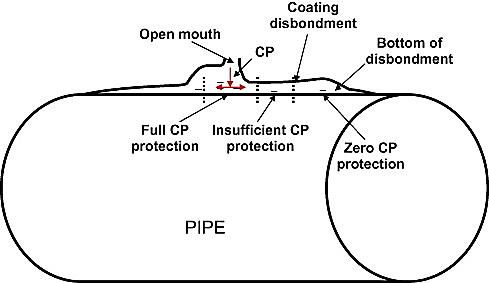
Selecting suitable coating for buried pipelines is one of the most important parts of protecting external surfaces and reducing the corrosion rate. Controlling corrosion through cathodic protection and specific coating reduces the cathodic current; however, this creates an alkaline environment in the interface of the coating that covers the cathode surface, which can lead to disbondment.
Disbondment of polyethylene-coated buried pipelines is one of the major problems faced by operators. In such cases, there is a risk of stress corrosion cracking (SCC). Suitable coatings and cathodic protection control corrosion of pipelines can increase the effectiveness of protection when used as a complementary system. Performance depends on the intrinsic properties of the coating and the metal surface.
Use of protective coatings reduces the cathode flow rate, but this current creates alkaline conditions under the boundary layer, and the metallic coating and increases the pH factor of the electrolyte at the interface of coating and metal. Eventually, this can lead to separation of the coating, or so-called cathodic disbondment.
Typically, a flaw in the coating starts to spread radially around the defect. Therefore, evaluation of the resisting cathodic current, underground pipeline coatings, surface preparation and design is crucial.
The first step in cleaning the surface cover is through thermal and chemical methods to remove the salts dissolved in water. Spray the surface with copper slag abrasive preparation and sand “rough fit” used to clean the surface. The role of protective coatings is important for pipelines. If there is not a proper coverage, serious threats for installations and pipelines can occur, as in Figure 1.
The lack of proper preparation is one reason for the weakness in the coverage, and the contaminants, such as dissolved salts, cause degradation of ion as well as blisters. Another problem is that hydrocarbons never allow proper adhesion of fusion-bonded epoxy coating.
Materials, Methods
Fusion-bonded epoxy (FBE) coating is a thermo-setting powder coating. FBE powder coatings are comparable to none. These consist of a combination of epoxy resins, epoxy curing agents, pigments, catalysts, fillers, flow agents and other additives. Epoxy powder coatings are applied by spraying compressed air to force the electrostatic field on the surface preparation.
An St52 steel substrate at a size of 6.4-mm-by-100-mm-by-100-mm prepared with copper slag and sandblasting methods has been readied at a standard of Sa 2.5 and the surface profile roughness has been evaluated.
To perform FBE coating, samples need to be preheated at 230°C for 10 minutes and the coating applied with an electrostatic system. Before coating, the surfaces are washed with 5% phosphoric acid for 20 seconds to remove all the contaminants.
Also, before coating, the metal needs to be preheated at 230°C for 10 minutes. Electrostatic spraying is done by varying the thickness of the sample applied. The samples are kept in the furnace to fully cure polymer cross-linking for five minutes at 200°C.
The cathodic disbondment test is performed, according to standard CSA Z245.20-02. A solution of 3.5% sodium chloride and distilled water is used as the electrolyte in all cells, and the platinum electrode is used as anode. Voltages of 1.5 and 3 are used at temperatures of 23°C and 65°C for 28-day and 48-hour test periods. The adhesive coverage around the cavity, created by the eight lines, are evaluated. To complete the curing process of polymer cross-linking, the samples are kept in the furnace for five minutes at 200°C.
Results, Discussion
Methods to discover the mechanism of the cathodic disbondment time is quickly expanding. An increase or decline in the expansion rate of the cathode area represents the separation of dominant cations in the process. If the rate of disbondment area increases over time, it points toward the cations guided through the coating to the separation. Most of the ions’ strength and low water absorption is provided by coating. (Figure 2).

In this article, the influence of 5% phosphoric acid, coating thickness and surface profile roughness for cathodic disbondment of oil and gas pipelines are evaluated. In the preparation, the following features are important to note:
• Density profiles influence the mechanical adhesion.
• Pollution has long-term negative effect on performance. So, remove all organic contaminants and soluble salts.
• Lower chloride levels results in increased strength and reduced cathodic disbondment. The purpose of washing after blasting abrasives (sand, copper slag) with phosphoric acid and ionized water is to increase adhering coating.
A sample surface roughness of 88 microns and coating thickness of 460 microns with a spraying copper has been covered with a minimum radius separation. The increase in thickness was followed for 28 days at 1.5 kV and a temperature of 23°C. The coating has a big influence on the separation process. In this 48-hour test, voltage was 3 and the temperature was 65°C. (Figures 3 and 4).

The role of surface preparation for apply fusion bonded epoxy coating is important because of reducing the contamination on lifetime coverage and occurring adhesion to the cathodic disbondment.
The results show the effect of increasing the thickness and surface preparation play an important role for protecting pipelines against corrosion and cathodic disbondment (Figures 3 and 4).

Conclusion
In general terms, the results can summarize in the following payments:
• The early prepared by spraying an abrasive material (sand, copper slag) surface can made according to standard ISO 8501 grade Sa 2.5.
• Profile of surface roughness plays an important role in adhering Fusion-Bonded-Epoxy coatings which cover the effective roughness between 70 to 100 micrometers. Increasing the surface roughness can reduce adhesion.
• Adhesion of coating on surface will increase by 5% phosphoric acid. In fact, the increase in viscosity because of forms a layer of iron phosphate change-over coating of stainless steel.
• Increasing the thickness of the coating improves strength and reduces cathodic disbondment while cathodic protection applied on oil and gas pipelines.
Author: Emad Behdad holds a master’s degree in materials and corrosion protection of materials from Najafabad University in the Isfahan Province of Iran.





Comments