March 2013, Vol. 240, No. 3
Features
Hot-Dip Galvanizing Vs. Zinc Electroplating
This article describes the two different process applications and quality-control aspects of zinc for corrosion control. A question often faced by engineers and material procurement personnel concerns making a choice between hot-dip galvanized material and zinc-electroplated options. The process, properties and quality-control issues of the two are discussed here in simple terms. This article also references typical applicable ASTM specifications.
In the oil and gas transmission industry, several materials are specified to be zinc-coated or zinc-electroplated to protect them and associated members of the structure from elements of environmental corrosion. The materials that are commonly specified to be zinc-coated or zinc-electroplated vary from structural members at the stations to some low-pressure pipelines and fittings within plants.
Galvanized fasteners, valves, flanges, structural materials for stations and several other civil construction materials are also specified to be either zinc-galvanized or zinc-electroplated primarily to prevent corrosion. The understanding of the differences in the two ways the zinc is applied and its quality control is essential in making proper selection and effective use of this versatile corrosion-control material. This article seeks to explain the differences with the references to the applicable specifications.
Galvanizing
A zinc coating, usually hot-dipped, in which the zinc and steel form a metallurgical bond. The thickness of a hot-dipped coating can be varied from a thin zinc/iron alloy layer to heavy applications suitable for extended outdoor exposure. Inline galvanized coatings are applied during the manufacturing process of the hollow or open section, with the cleaned steel section exiting the mill and passing into the galvanizing bath. This coating is usually measured as thickness or as coating mass in grams per square meter and ranges from a minimum of about 100 g/m2 upwards, with an average around 175 g/m2.
ASTM A 123 is the specification for Hot Dip Galvanizing. This specification covers the standard requirements for hot-dip galvanized zinc coatings on iron and steel products made from rolled pressed and forged shapes, castings, plates, bars, and strips. This specification deals with unfabricated products and fabricated products, for example, assembled steel products, structural steel fabrications, large tubes already bent or welded before galvanizing, and wire work fabricated from uncoated steel wire.
Also covered in this specification are steel forgings and iron castings incorporated into pieces fabricated before galvanizing or those too large to be centrifuged (or otherwise handled to remove excess galvanizing bath metal). ASTM A 123-90 supercedes earlier versions of ASTM A 123 and ASTM A 386. Reinforced steel bars are galvanized in accordance with ASTM A 767/A 767M.
Inspection And Testing
Following ASTM specifications comes in handy for inspecting and testing various stages of galvanizing process.
ASTM E 376: Practice for Measuring Coating Thickness by Magnetic Field or Eddy Current (Electromagnetic) Examination Methods.
ASTM A780 Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coating.
ASTM A 90: Test Method for Weight (Mass) of Coating on Iron and Steel Articles with Zinc or Zinc-Alloy Coatings.
ASTM A 902 Terminology Relating to Metallic Coated Steel Products.
ASTM B 487: Test Method for Measurement of Metal and Oxide Coating Thickness by Microscopical Examination of Cross Section
ASTM B 6: Specification for Zinc.
ASTM B 602: Test Method for Attribute Sampling of Metallic and Inorganic Coatings.
Continuous Galvanizing Grade
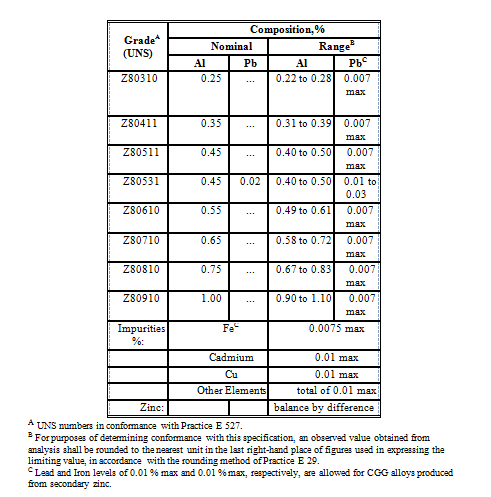
ASTM B 852 covers grades of zinc alloys, commonly known as continuous galvanizing grade (CGG) alloys, that contain aluminum, or aluminum and lead and that are used in continuous hot-dip galvanizing of steel sheet. CGG alloys are tested and conform to the chemical composition requirements as determined by chemical analysis on samples taken. CGG alloy castings should be free of undue surface oxide, adhering foreign matter, and any flash that would interfere with handling and use. Samples obtained during casting, drilling or sawing are analyzed individually and the average of the individual samples used to determine the analysis values for the lot.
Zinc Electroplating
This is also called zinc coating, but applied in a cold, electrolytic bath rather than a molten zinc bath. Traditionally the plating/coatings are thinner than hot dipped and not suitable for extended outdoor exposure. In this process a layer of pure zinc is applied. The thickness of plating ranges from a few microns on cheap hardware components to 15 microns or more on good-quality fasteners. Technical and cost issues prevent the economical plating of components with heavier coatings.
Electroplating Process
This is a plating process that uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal. Electroplating is primarily used for depositing a layer of material to bestow a desired property like corrosion protection. The process used in electroplating is called electrodepositing; the process is analogous to a galvanic cell acting in reverse.
The part to be plated is the cathode of the circuit. In one technique, the anode is made of the metal to be plated on the part. Both components are immersed in a solution called an electrolyte containing one or more dissolved metal salts as well as other ions that permit the flow of electricity. A rectifier supplies a direct current to the anode, oxidizing the metal molecules that comprise it and allowing them to dissolve in the solution.
At the cathode, the dissolved metal ions in the electrolyte solution are reduced at the interface between the solution and the cathode, such that they “plate out” onto the cathode. The rate at which the anode is dissolved is equal to the rate at which the cathode is plated, vis-a-vis the current flowing through the circuit. In this manner, the ions in the electrolyte bath are continuously replenished by the anode.
Zinc metal has a number of characteristics that make it well-suited for use as a coating for protecting iron and steel products from corrosion. Its excellent corrosion resistance in most environments accounts for its successful use as a protective coating on a variety of products and in many exposure conditions. The excellent field performance of zinc coatings results from their ability to form dense, adherent corrosion product films and a rate of corrosion considerably below that of ferrous materials, some 10 to 100 times slower, depending upon the environment. While a fresh zinc surface is quite reactive when exposed to the atmosphere, a thin film of corrosion products develops rapidly, greatly reducing the rate of further corrosion. The following figure shows the expected service life to first maintenance (5% red rust) of iron and steel, based on the zinc coating thickness and the environment.
ASTM B633 – 07 Standard Specification for Electrodeposited Coatings of Zinc on Iron and Steel.
This specification establishes the requirements for electro-deposited zinc coatings applied to iron or steel articles for corrosion-protection purposes. The coating is provided in four standard thickness classes in the as-plated condition or with one of three types of supplementary finishes. The surface of the article is cleaned as pre-plating basis metal, pre- and post-coating treatment is given to reduce the risk of hydrogen embrittlement. Coating is sampled, prepared, tested and conform accordingly to this specification the general criteria is the appearance (luster and workmanship), thickness, adhesion, corrosion resistance, and hydrogen embrittlement.
High-strength metals, unless otherwise specified, including high-strength steels having a tensile strength greater than 247 ksi or hardness ? RC 46 are not electroplated.
Stress relieving of all parts with ultimate tensile strength ?145, 000 psi at minimum 375°F for three hours or more is recommended.
If these facts are considered and adhered to in specifying and ensuring that the vendor complies with the process and follows with the test results, the desired level of corrosion protection can be achieved.
Author
Ramesh Singh, is Senior Principal Engineer (Materials, Welding and Corrosion at Gulf Interstate Engineering in Houston. He can be reached at (713) 850-3687 or at rsingh@gie.com.





Comments